The giant American liver fluke is a flatworm that occurs in various ruminants, using them as its definitive hosts. While some of these host species largely tolerate infections with the fluke, others react very sensitively to it.
The GAL was detected on the Bavarian side of the Böhmerwald/Šumava ecosystem for the first time in autumn 2019. From April 2021 to December 2022, the Bavarian State Institute of Forestry investigated the infectation situation on the spot, in cooperation with its project partners, the Bavarian Forest National Park, the Šumava National Park and the Neureichenau forest enterprise (Bavarian State Forests).
The focus was on surveying the current infection rates in red deer, roe deer and wild boar. The distribution of intermediate and definitive hosts was also surveyed, in order to localise possible infection hotspots of the giant American liver fluke.
Introduced with wild animal imports
Unlike its relative the large liver fluke (Fasciola hepatica), the giant American liver fluke (Fascioloides magna; GAL) is not an indigenous parasite. It probably reached the European continent from North America in the second half of the 19th century, with wild animals imported for breeding in enclosures. It was first recorded in 1875 in a red deer from the Royal Park of La Mandria, near Turin in Italy. Further accidental introductions have allowed GAL to establish three stable populations in Europe: in the area near Turin, in the Czech Republic/south-west Poland, and in the alluvial forests along the River Danube.
As early as the 1930s, individual cases of GAL were also reported in Germany. From the year 2000 onwards, there have been increasing reports of the parasite in south-western Bohemia (Czech Republic), and since 2010, there have been more and more cases in north-eastern Bavaria. The first evidence of the parasite on the German side of the Bohemian Forest ecosystem came from a red deer shot in the Bavarian Forest National Park in autumn 2019.
The various host species originally infected on the American continent, such as white-tailed deer or wapiti deer, do not occur naturally in Europe. In their absence, the GAL attacks species native to Europe - particularly the red deer. The GAL also parasitises many other freely roaming ungulate species, including fallow and sika deer, roe deer, chamois and wild boar. Cattle, horses, and pig, goat and sheep herds are however also affected. Some of these hosts belong to the so-called secondary or accidental hosts (see info box). Leeches that use hosts like these do not usually succeed in reproducing. The life cycle of the GAL is complex and includes a semi-aquatic snail as an intermediate host as well as the final host.
The parasite changes its host

Fig. 2: Life cycle of the giant American liver fluke: 1. Adult fluke in the liver of a definitive host, 2. Egg, 3 Mirazidium, development via sporocyst to mother and daughter rediae in intermediate hosts 4. Radix labiata freshwater snail or 5. Galba truncatula freshwater snail, 6. Cerkaria, 7. Metacercaria. Source: Pavel Procházka
The life cycle of the GAL takes place in two hosts and includes numerous larval stages (Figure 2). The adult fluke produces a large number of eggs in the liver of a specific final host (see info box). These enter the host's intestine via the bile ducts and are excreted. In a humid environment, a larva develops in the parasite egg. The free-swimming ciliated larva (mirazidia) hatches after a few weeks depending on the ambient temperature, and is very short-lived. Using light stimuli and chemical signals, it has to find a suitable intermediate host within a few hours. In Europe, the GAL uses two semi-aquatic snail species from the pond snail family (Lymnaeidae) as intermediate hosts: the Galba truncatula freshwater snail or, more rarely, the Radix labiata freshwater snail.
The parasite larva penetrates into its intermediate host via the outer skin and develops into a sporocyst. Several divisions now take place in the snail: more than 1,000 cercariae can develop from a single sporocyst via mother and daughter rediae. These emerge from the snail and attach themselves as cysts to plant components. Low temperatures slow down the development process, but do not always lead to the death of the parasite. Parasite eggs or the developmental stages in the snail can thus survive the winter and continue their development the following spring.
The microscopic cysts contain the metacercariae, which are infectious for the definitive host. In a moist environment, they can survive in the cyst for several months, but in a dry environment they die after a few weeks. The definitive host becomes infected by ingesting contaminated plant material. The metacercariae lose their protective coating in the digestive tract of the definitive hose, and penetrate the intestinal wall to enter the abdominal cavity of their host. From there, they reach the liver. The GAL now migrates into the tissue of the liver, creating feeding tunnels as it goes. This causes damage to the liver. The disease symptoms vary in severity, depending on the intensity of the infestation. Some host species - such as the roe deer - react particularly sensitively, which is why they may die from even a moderate infestation. The disease pattern or collective symptoms caused by GAL is called fascioloidosis.
The migratory behaviour of the GAL in the host liver usually ends when the parasite encounters conspecifics. As soon as this happens, the host's immune system starts to form a fibrous tissue capsule called a pseudocyst. This usually results in the encapsulation of several flukes together. In the pseudocyst, the flukes grow to a size of about 35 x 100 mm and reach sexual maturity. The hermaphrodite flukes reproduce sexually and produce many eggs. Flukes that do not encounter a conspecific in their host's liver can be encapsulated individually in a cyst and reproduce asexually. In specific end hosts (see info box), the pseudocysts are connected with the intestine of the end host via the bile ducts. This is how the parasite eggs are passed out into the environment with the host's faeces.
The liver fluke and its hosts surveyed
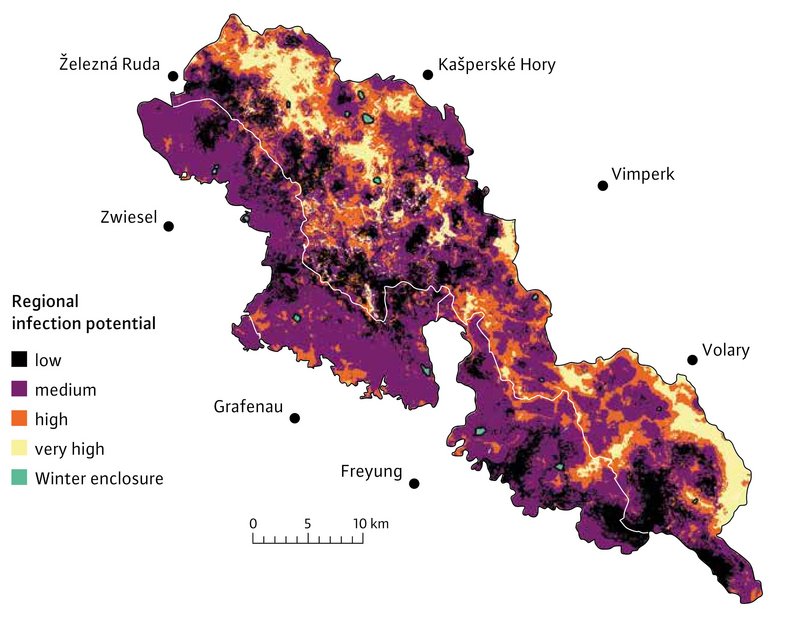
The two snail species used by the GAL as intermediate hosts were mapped at 865 sites in the study area in summer 2021, and their occurrence was analysed in relation to environmental parameters. Based on photo data from game cameras and with the help of various environmental variables, distribution maps were created for red deer, roe deer and wild boar. This allowed us to generate prognoses for the occurrence of all relevant intermediate and final host species across large areas. In the project area, the GAL mainly uses red deer for reproduction. By blending the distribution maps, it was possible to identify the areas in which the GAL encounters suitable intermediate hosts, and which are also used by red deer (Figure 3). It can be assumed that it is mainly in these areas (infection hotspots) that end hosts become infected with GAL.
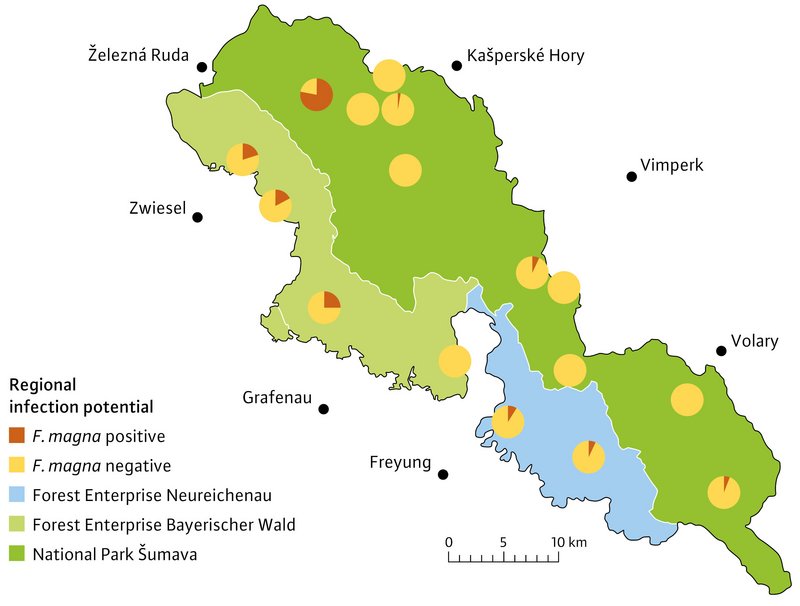
The distribution of Fascioloidosis in the study area was estimated using two different methods. In the first method, faecal samples were collected in the winters 2020/21 and 2021/22 from all red deer winter enclosures, and analysed for liver fluke eggs. GAL was detected in nine of the 16 winter enclosures. The “Zadni Chalupy” enclosure in the north-west of the Šumava National Park showed a particularly high density of GAL-positive faecal samples (78 %) (Figure 4).
The second method was the examination of the livers of red deer, roe deer and wild boar killed by hunters in the hunting year 2021/22. Advanced infestations could be reliably diagnosed with the help of this method. The infection rate in red deer of all age categories was highest in Šumava National Park, at almost 17.8% (Figure 5). In the Bavarian Forest National Park and in the Neureichenau forest enterprise, the infection rates were somewhat lower, at just under 14.5% and 12.4% respectively. Males were less often infected than females here, which may be due to differences in spatial use behaviour. With increasing age, the proportion of individual red deer infected increased. The highest rate of infection, at just under 35.3%, was found in the female adult animals from the Bavarian Forest National Park. In red deer calves, infestations were only detected in isolated cases. Liver samples from roe deer and wild boar were taken only in the Šumava National Park and in the Neureichenau forest enterprise. An infestation was diagnosed in just one of the 155 roe deer livers examined. Of the 482 wild boar livers examined, none was infested.
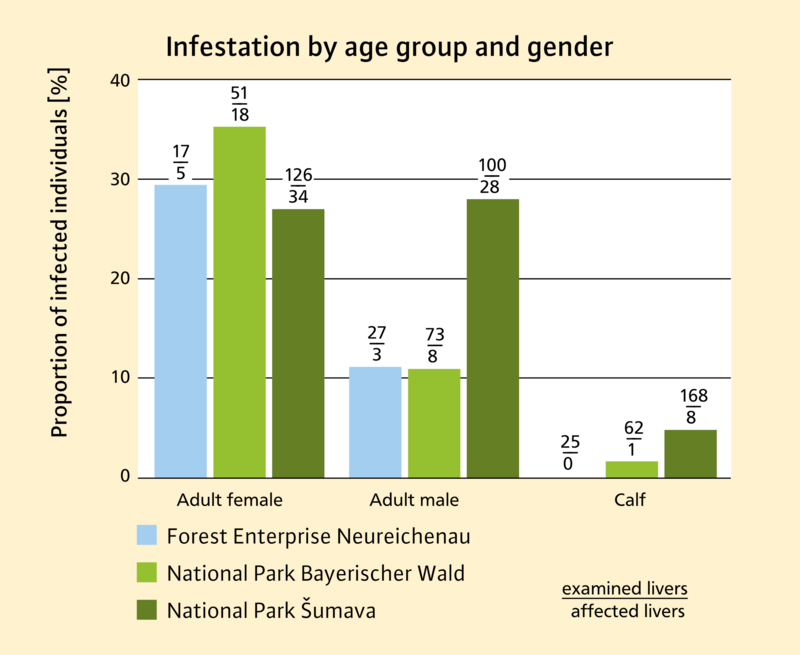
Fig. 5: Percentage of red deer infected with the American liver fluke in the individual parts of the project area, differentiated according to sex and age class. The boxes on the bars contain the total number of livers examined and the number of livers infested with the American liver fluke. Source: Tomáš Peterka (Šumava NP)
Containing the spread
The possibilities for containing the spread of GAL are limited. The few available options for action start at different points in the complex life cycle of the parasite. What they have in common is that they require close cooperation between local councils, farmers, hunters and hunting and wildlife management organisations.
One way of protecting grazing animals is to fence off areas that are a suitable habitat for the intermediate hosts of GAL. The erection of wildlife-proof fences can also serve to protect grazing animals. However, the fences are not a barrier for the parasite larvae or infected intermediate hosts, so that this measure is only suitable for reducing the risk of infection. Farmers and wildlife managers can further minimise the risk of infection by feeding their animals only thoroughly dried hay that has been stored for at least a few weeks. Another possibility for regional containment of GAL is to ensure potential host animals are subject to a thorough veterinary examination before transport. If an infection is detected, further spread can be effectively prevented by administering medication.
The containment of GAL infections in wildlife, on the other hand, is much more difficult: Medicating animals in the wild is complicated and often impossible. Controlling the exact dosage of medication through additional feed is only possible to a limited extent where the animals roam freely, and especially when the species form herds. Medication against fascioloidosis was administered at feeding stations and in the winter enclosures in the Šumava National Park for years, but with no lasting effect, so that the administration of the drug was discontinued. Drug residues in the excreta of treated animals can also have an effect on the soil fauna. Such measures should thus not be applied generally in protected areas.
A lot of droppings are deposited over a small area in winter in the red deer winter enclosures. Infected animals bring more parasites into the habitat here. With rising temperatures in spring, parasite larvae develop from the eggs. Within the enclosures, the wild animals also always have access to water. At the same time, the water offers a suitable habitat for the intermediate hosts of the GAL. The conditions for the parasite in the enclosures are thus particularly favourable. Wild animals that also use the winter enclosures in summer are thus exposed to an increased risk of infection. Closing the winter enclosures over the summer could protect these animals from infection and thus reduce infection pressure on red deer.
The project findings represent a momentary snapshot of the infection situation. Since it can be assumed that the spread of the parasite is dynamic at the moment, further monitoring of the occurrence of infections is essential. However, such monitoring should not only focus on one game species, but include all potential host species. It is particularly important to include the roe deer, which reacts very sensitively to GAL infestation.
Summary
As part of the INTERREG project, a survey was carried out to assess the infection situation of red deer, roe deer and wild boar with the invasive parasite “giant American liver fluke (GAL)” in the Bohemian Forest ecosystem. The modelled distribution maps of intermediate, final and aberrant hosts then allowed the localisation of infection hotspots for final and aberrant hosts. In order to estimate the distribution of GAL in the project area, faecal samples from 16 winter enclosures were examined - GAL was detected in nine enclosures. The examination of wild animal livers revealed infection rates of between around 12% and 18% for red deer. Roe deer and wild boar do not play a role in the current infection dynamics. One way to positively influence the infection situation is to close the red deer winter enclosures over the summer. Follow-up monitoring should be encouraged.
The project “Assessment of risk to wildlife presented by the invasive parasite giant American liver fluke” (Running time: April 2021 - December 2022) was mainly financed by the Bavaria-Czech Republic Cross-Border Cooperation Programme Objective ETC 2014-2020 (INTERREG V).
Info box: Definitive hosts of the giant American liver fluke (GAL)
The classification of the definitive hosts depends on their suitability as a host for completion of the parasitic life cycle.
- Specific definitive hosts
- Reproduction of the GAL possible
- Representatives of the Cervidae family in Europe: Red deer (Cervus elaphus), fallow deer (Dama dama), sika deer (Cervus nippon)
- Infestation with single parasites is usually asymptomatic; with heavy infestation weight loss is possible, and in male hosts antler formation may be impaired; death only with extreme infestation
- Secondary hosts
- Immune system forms pseudocysts in the liver, but these are thick-walled and rarely connected to the intestine via bile ducts; parasite eggs produced consequently cannot leave the pseudocyst
- Examples: Domestic cattle (Bos taurus), domestic horse (Equus caballus), domestic pig(Sus scrofa f. domestica), wild boar (Sus scrofa)
- Similar disease symptoms to the specific end hosts, infections in cattle almost always clinically inconspicuous
- Accidental hosts
- No formation of pseudocysts
- Roe deer (Capreolus capreolus), domestic goat (Capra aegagrus f. hircus), domestic sheep (Ovis gmelini f. aries), chamois (Rupicapra rupicapra)
- Particularly at risk from infestation, as the constant migratory behaviour of the fluke in the liver significantly damages the liver; even minor infestations can lead to death within a few months if left untreated